Time:2022-07-28 Views:2
American Society of Clinical Oncology (ASCO) JournalJournal of Clinical Oncology" (JCO) recently published an important large-scale study of nearly 6,000 patients with relapsed and refractory tumor genomes and potential matches< a href="https://www.zhihu.com/search?q=%E9%9D%B6%E5%90%91%E7%96%97%E6%B3%95&search_source=Entity&hybrid_search_source=Entity& ;hybrid_search_extra=%7B%22sourceType%22%3A%22answer%22%2C%22sourceId%22%3A1526720754%7D" data-ke-src="https://www.zhihu.com/search?q=%E9%9D%B6%E5%90%91%E7%96%97%E6%B3%95&search_source=Entity&hybrid_search_source=Entity& ;hybrid_search_extra=%7B%22sourceType%22%3A%22answer%22%2C%22sourceId%22%3A1526720754%7D" target="_blank" class="css-pgtd2j">targeted therapyIn-depth analysis gives usa new understanding of precision cancer treatment options.
"We worked hard to list All potential drugs have been established for the use of next-generation sequencing in clinical trials New benchmark.Over time, It will be more efficient to conduct extensive clinical research based on tumor genetic testing results.”
One of the authors of the paper,University of PennsylvaniaTumor "The study describes the genetic complexity unique to relapsed and refractory cancers," said Peter J. O'Dwyer, Ph.D., a research scientist. "This paper represents a translation of our understanding of cancer genetics into Important milestone for better treatments.”

 Screenshot source: Journal of Clinical Oncology
Screenshot source: Journal of Clinical Oncology
5 years ago, the ECOG-ACRIN Cancer Research Group and the National Cancer Institute (NCI) launched the "NCI-Molecular Analysis of Therapy Choice (NCI-MATCH)" study.
This is a signal discovery experiment. The goal is to match, in relapsed and refractory patients, not limited to cancer types, but to the genetic abnormalities known to drive tumors, to find potentially effective targeted therapies.
Specifically, the researchers recruited a total of 5954 patients at approximately 1117 clinical centers across the United States, and collected their Tumor samples were then pooled for genomic testing by next-generation sequencing and assigned to 30 different treatment groups based on the results. Some of these therapies have shown potential in other clinical trials, and some have received FDA approval in at least one cancer type.
 ▲NCI-MATCH study aims to find potentially effective targeted therapy for patients with relapsed and refractory cancer. (Image credit: ECOG-ACRIN Cancer Research Group)
▲NCI-MATCH study aims to find potentially effective targeted therapy for patients with relapsed and refractory cancer. (Image credit: ECOG-ACRIN Cancer Research Group)
According to the research team, "for patients with rare cancers who have progressed after one or more standard treatments and for whom treatment is lacking, this is the largest clinical trial to date. Large-scale precision medicine trials. "
1. In the trial, nearly 40% of patients with genetic abnormalities have matched targeted therapy
In the NCI-MATCH trial, 93% of the samples had analyzable genetic test results, and 37.6% of the mutations had matchable results The potentialtargeted drugs. Despite the limitations of the trial protocol, patient histology, etc., the proportion of patients who actually received matched treatment was 17.8%.
thisMatch rateOnly 30 drugs were included in this trial, and this percentage may increase as treatment options expand. This finding means that genetic testing of tumors is extremely valuable, and patients have a nearly 40% chance of finding potential targeted treatments.
2. The odds of targeted therapy vary by cancer type
To the team's surprise, patients with rare cancers had the highest rate of matches between detected mutations and potentially targeted drugs . CNS cancer (37.2%), urothelial carcinoma (36.0%), cholangiocarcinoma(25.9%) , cervical cancer (28.4%), Gastroesophageal cancer (27.8%), melanoma (26.3%), uterine cancer (26.2%) and anal cancer< /a>(25.5%), more than 25% of patients received experimental treatment that matched the tumor gene defect .
By contrast,pancreatic cancer and small cell lung cancer, the numbers were only 5.8% and 5.1%, respectively.
In breast cancer,colorectal cancer< /span>, non-small cell lung cancer and prostate cancer are four common cancers, the actual experimental treatment The average match rate is only 16.4%.
3. It is common for a patient to carry multiple oncogene abnormalities at the same time
In the trial population, the most common deleterious or activating mutations observed by the researchers wereTP53(47.4%), KRAS (21.2%), and APC(12.4%). The most commonly observed mutation that can be matched to targeted therapy isPIK3CA< /i>(11.8%) and PTEN (6.3%) .
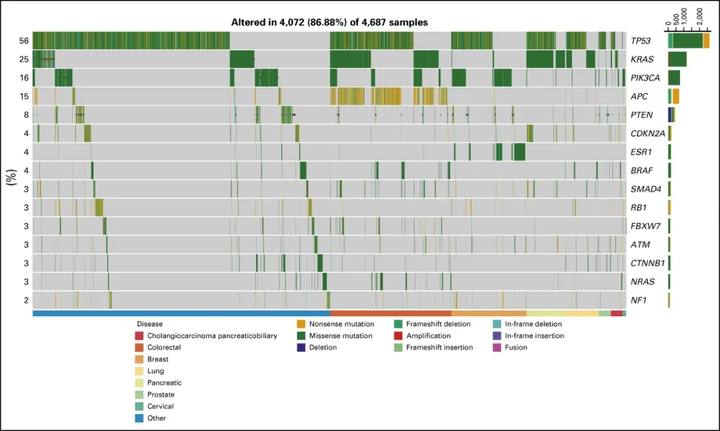
 ▲The distribution of patient gene alterations observed in the NCI-MATCH trial (Image source: Reference [1])
▲The distribution of patient gene alterations observed in the NCI-MATCH trial (Image source: Reference [1])
52.8% of tumor samples had both mutations. The most common co-occurring mutation is KRASandTP53.
This finding suggests that the industry should explore combinations of therapies that simultaneously target multiple genetic defects.
4. Cancer-causing mutations do not change much over time and treatment regimens
The researchers compared the genetic makeup of tumors in patients with seven cancer types to The Cancer Genome Atlas (TCGA), which includes Data from a large number of patients with untreated primary tumors. The seven cancers included breast, bile duct, uterine, colorectal, lung, pancreatic and prostate cancers.
Comparison found that, overall, the 7 mutations in patients with metastatic cancer, and patients with untreated primary tumors mutation, there is not much difference.
Although this finding came as a surprise to the researchers, it is still insufficient due to the lack of genetic databases of other metastatic cancer patients in the industry. to draw conclusions. Next, the researchers plan to compare mutational information in patients' primary and metastatic tumors in NCI-MATC.
In addition, in the NCI-MATCH cohort, TP53Mutation andRASmutation frequencies are higher. This is consistent with what has been observed in other advanced cancer populations, but the mutations were not associated with the amount of prior treatment the patient had. After receivingEGFRInhibitor-treatedEGFRin nearly half of patients with mutant non-small cell lung cancer span>EGFR T790Mmutation.

Image source: 123RF
At present, NCI-MATCH is also expanding the available targeted drugs for patients, which has increased to 38 treatment groups. Data from each treatment arm will inform which cancer types the regimen is more effective against, and potential treatments will then enter larger, more targeted trials. It is believed that this information will lead to more insights as future treatment outcomes for each group accumulate.
Phone: 400-099-1215
Company address: Tide Valley Biomedical Industrial Park, Xinxing Park, Gu'an County, Langfang City, Hebei Province
Enterprise Email: boya@newcby.com
New Century Elite Life Technology (Hebei) Co., Ltd. Copyright(C)2021 Ji ICP preparation ******* No.-1